What are Humic Acids?
1. Humic matter
Humic matter is formed through the chemical and biological humification of plant and animal matter (pic.1.1) and through the biological activities of microorganisms.
Picture 1.1 — Typical vegetation of the Tertiary period.
The best source of humic acids are the sedimentation layers of soft brown coal, which are referred to as Leonardite. (pic.1.2, pic.1.2a, pic.1.3) Humic acids are found in high concentration here.

Pic.1.2 - Carbonisation process (moor→ peat → (Leonardite) → lignite).
(Copyright © Humintech 2018)
Pic.1.2a - Typical vegetation of the Tertiary period

Pic.1.3 - Remains of a sequoia trees of the Tertiary period (found in the coal-mine Donatus, West Germany in 1907).
The scientific research of humic acid and their usable properties has a long tradition in Germany, which began with the works of the chemist Franz Carl Achard (1753-1821). The commercial employment of brown coal containing humic acid e.g. as coloring material under the designation “Cologne brown” from Cologne lignite mining area and “Kassler brown” goes back to 19th century in Germany. In the course of the last century the use of humic acid has been established in the agricultural, medical and environmental sector. Humintech is a German technology enterprise, based in Grevenbroich, which ties in with this longtime tradition. We are mining up to 70 million year old raw material. Germany is by far the worldwide leading producer of lignite with 176 million tons, followed by China with about 100 million tons and the USA with 75 million tons. Our experienced geologists select the mining areas of the well-known outstanding German lignite sources with respect to a low pollutant content and a high oxidation degree,
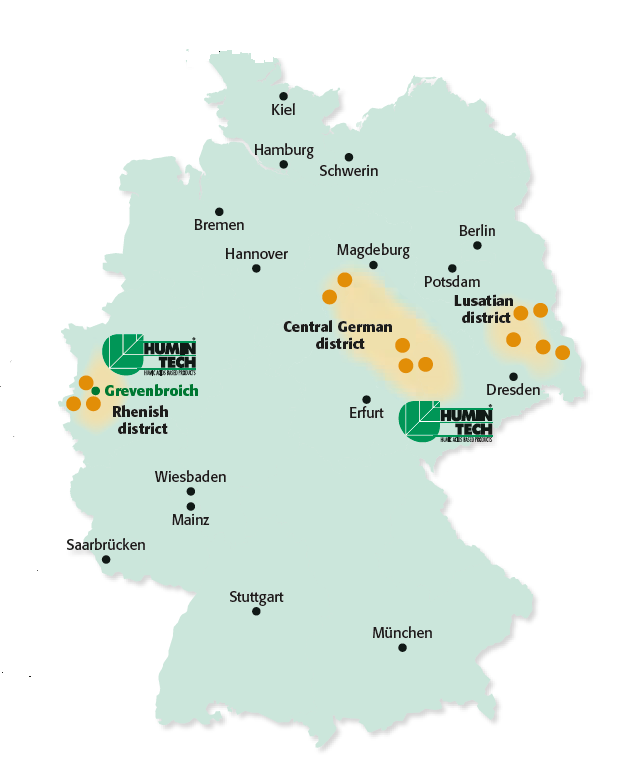
which are of crucial importance for the bioactivity of the raw material. Since the beginning of the 70’s former open cast lignite mining fields were recultivated in Germany. Since then we produce and develop humic acids based products for agriculture and other sectors. During production processes we pay particular attention to careful processing of the raw materials, in order to receive their bioactive structures. Our experience of many years and patented procedures are a guarantor for the unique quality of our products, which is confirmed again and again by our customers worldwide. Our products are exported in over 70 countries at retailers and formulators and we are ranked today among the leading producers and exporters of humic acids based products.
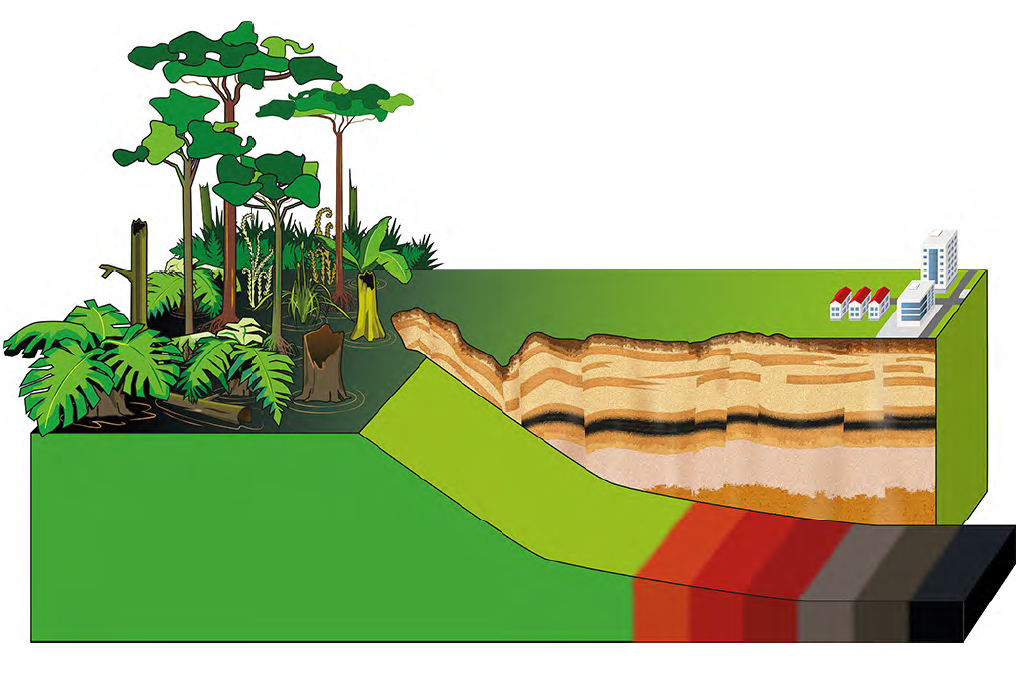 Coal formation: (from bog via Lignite to coal)
Coal formation: (from bog via Lignite to coal)
Coal formation: from bog via Lignite to coal. (Copyright © Humintech 2018)

Examination of a coalbed at the lignite mining strip site Garzweiler II, Germany.
 Examination of a coalbed at the lignite mining strip site Garzweiler II, Germany
Examination of a coalbed at the lignite mining strip site Garzweiler II, Germany
Garzweiler II, lignite mine, Germany

Garzweiler II, lignite mine, Germany

Garzweiler II, lignite mine, Germany
Headquarters Humintech, Germany.
Humic acids and their sources
Humic substances can be found in all soils and waters and arise from vegetable decomposition products. They are split up by extraction in humin, humic acid and fulvic acid. Their salts are called humates and fulvates. As main fraction humic acid forms the biological center of the humus. A fertile soil contains maxi-mal 3 % and peat of about 3-10 % humic acid. In a certain layer of soft brown coal, which did not reach the stage of lignite yet, humic acid can be found in a concentration up to 85 %. This soft brown coal layer is internationally called Leonardite. Leonardite differs from soft brown coal by a higher oxidation degree and its higher content of humic acid. Since the detection of high humic acid contents in Leonardite their commercial production for the agriculture increased drastically.
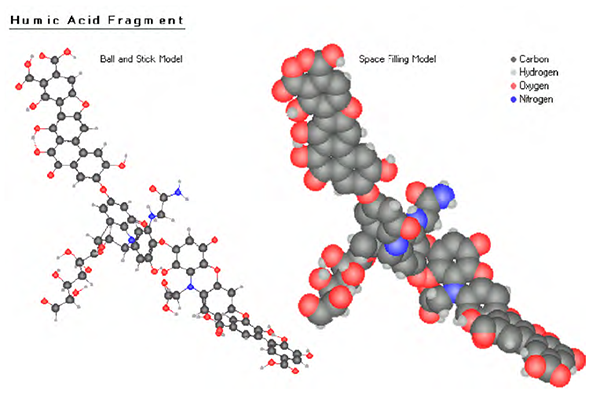
The biological center, the main fraction of natural humic matter, are the humic acids, which contain humic acid and fulvic acid. (fig.1.1, pic. 1.4, 1.5). Humic acids are an excellent natural and organic way to provide plants and soil with a concentrated dose of essential nutrients, vitamins and trace elements. They are complex molecules that exist naturally in soils, peats, oceans and fresh waters.
Leonardite is organic matter that has not reached the state of coal. It differs from soft brown coal by its high oxidation degree as well as its high content of humic acids carboxyl groups. Fig. 1.2 shows the chemical extraction of leonardite.
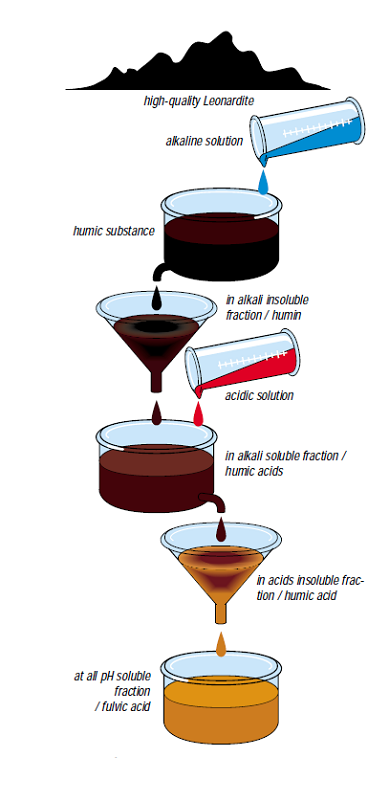
Figure 1.2 — Isolation of humic acid and fulvic acid by Achard (1786).
(Copyright © Humintech 2018)
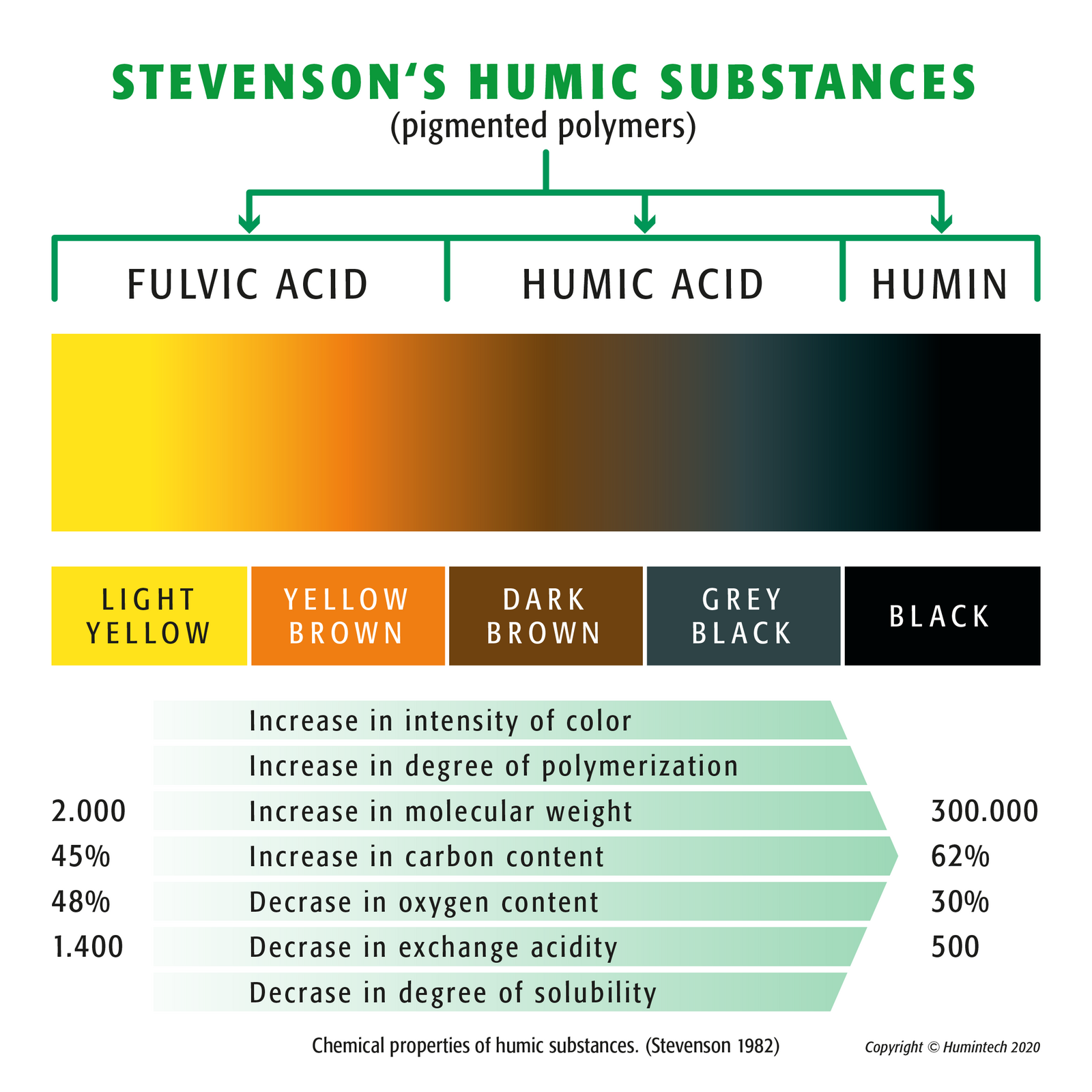
Stevenson Humic Substances (pigmented polymers) - (Copyright © Humintech 2020)
Shows the formation of coal (lignite) to Leonardite and its basic extraction to obtain
water-soluble humic acids (Rausa et al.). (Fig.1.3)
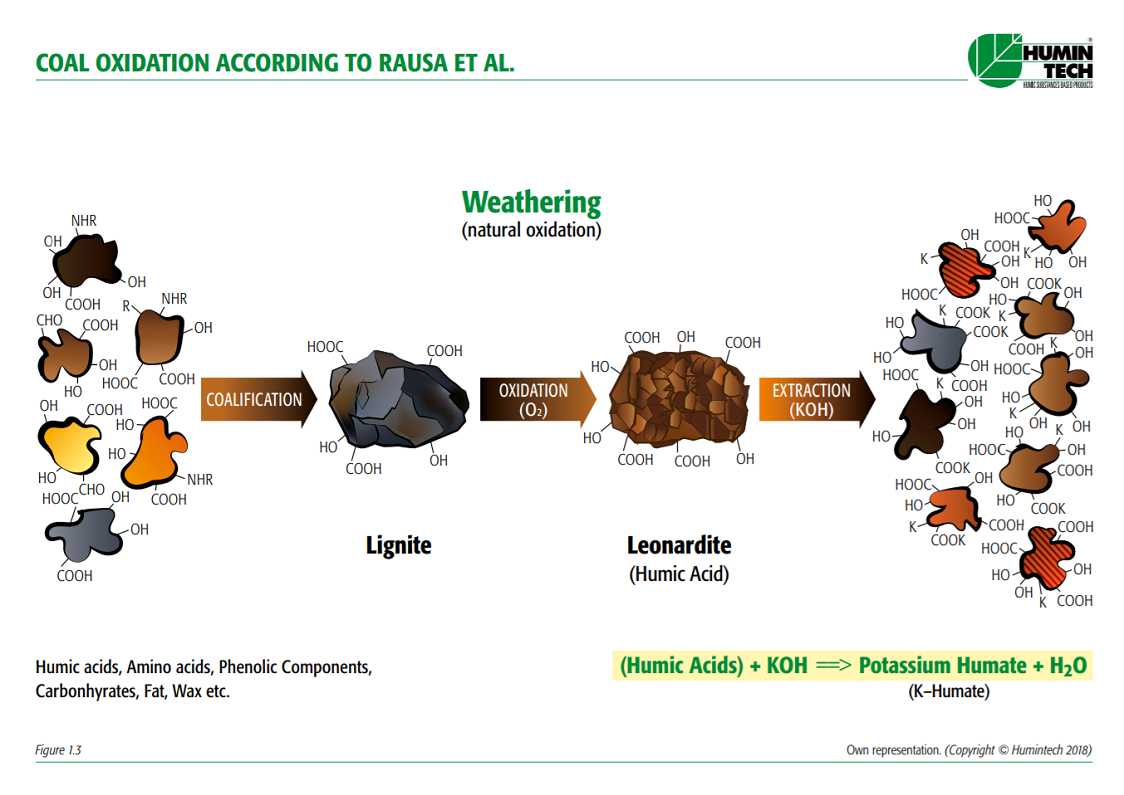
Figure 1.3 — Coal oxidation according to Rausa et al. (Copyright © Humintech 2018)
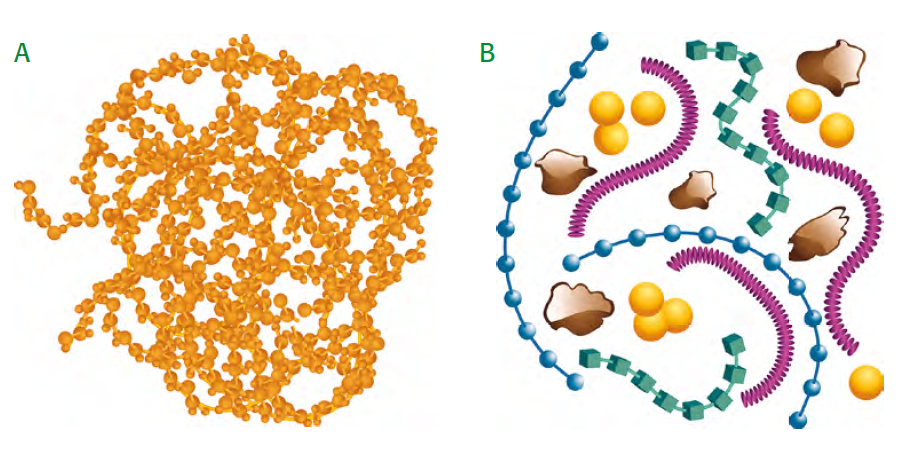
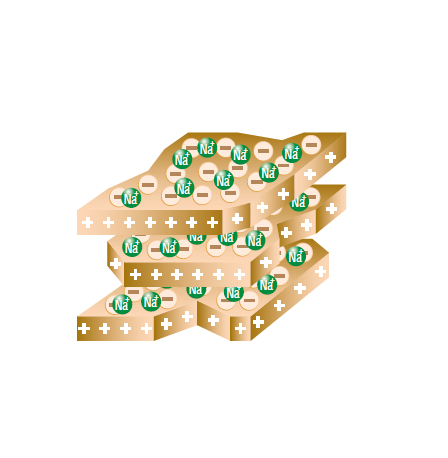
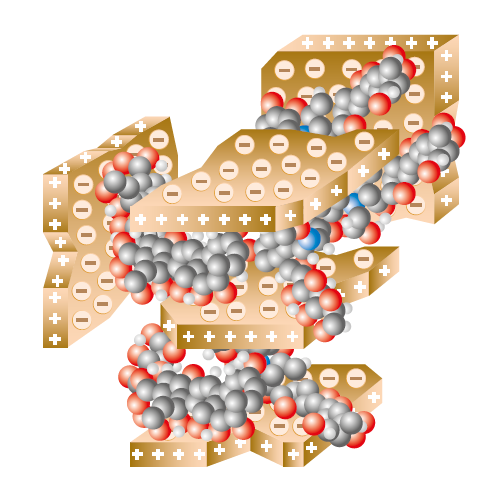
According to contemporary of view, Humus does not consist of long-chained humic substances (fig 1.4 A), but of short-chained chemical substances of different kind (fig 1.4 B), which build aggregates with cations (yellow) and clay particles: polysaccharides (blue), polypeptides (green), aliphatic groups (e.g. fats) (bordeaux), aromatic lignin fragments (brown) (source: Simpson et al., 2002).
Compared to other organic products, Leonardite is very rich in humic acids. While Leonardite is the end product of a humification process lasting 70 million years, the formation period of peat, for instance, is completed within only a few thousand years.
Therefore, Leonardite and other sources of humic acids differ in their molecular structure, which elucidates the extremely bioactive properties of Leonardite. This biological activity is about five times stronger than other humic matter. One kilogram of Leonardite corresponds to about five kilogram of other organic sources of humic acids.
In terms of humic acid content, one liter of LIQHUMUS® (liquid concentrate, pic.1.6, 1.7)
is equivalent to 7– 8 metric tons of organic manure.
 Liquid concentrate of water-soluble humate
Liquid concentrate of water-soluble humate
Picture 1.6 — Liquid concentrate of
water-soluble humate.
(Copyright © Humintech 2018)
Picture 1.7 — LIQHUMUS®
(Copyright © Humintech 2018)
Similarly, one kilogram of POWHUMUS® (concentrated powder, pic. 1.8, 1.9) is equivalent to about 30 metric tons of manure. However, Leonardite is not a fertilizer.
 Concentrated powder of potassium humate
Concentrated powder of potassium humate
Picture 1.8 — Concentrated powder
of potassium humate.
(Copyright © Humintech 2018)

Picture 1.9 — POWHUMUS®
(Copyright © Humintech 2018)
Leonardite acts as a conditioner for the soil and as a biocatalyst and biostimulant for the plant (pic. 1.10). Compared to other organic products, Leonardite enhances particularly plant growth (biomass production) and fertility of the soil.
Another advantage of Leonardite is its long-term effectiveness, as it is consumed as quickly as animal manure, compost or peat. As Leonardite is a degradation product, it does not compete with plants for nutrients such as nitrogen. This is not the case with incompletely decomposed compost, whereby the organic substances in soil are rapidly consumed up by micro organisms and mineralize without any humus formation.
Our Leonardite-based products improve the soil structure for up to five years.

Picture 1.10 — Development of root system of Tomato plant with LIQHUMUS® (right) and with water only (left). (Copyright © Humintech 2018)
2. BENEFITS OF HUMIC ACIDS
Current scientific studies show that the fertility of soil is determined to a very large extent by the content of humic acids. Their high cation-exchange capacity (CEC), the oxygen content as well as the above average water holding capacity are the reasons for the high value of using humic acids for improving soil fertility and plant growth.
The most important feature of humic acids is their ability to bind insoluble metal ions, oxides and hydroxides, and to release them slowly and continually to plants when required. Due to these properties, humic acids are known to produce three types of effects: physical, chemical and biological.
2.1. Physical Benefits:
Humic acids physically modify the structure of the soil. They
- improve the structure of soil: Prevent high water and nutrient losses in light, sandy soils. Simultaneously convert them into fruitful soils by way of decomposition. In heavy and compact soils, aeration of soil and water retention are improved; cultivation measures are facilitated.
- prevent soil cracking, surface water runoff and soil erosion by increasing the ability of colloids to combine.
- help the soil to loosen and crumble and thus increase aeration of soil as well as soil workability.
- increase water holding capacity of soil and thus help resist drought.
- darken the color of the soil and thus help absorption of the sun energy.
2.2. Chemical Benefits:
Humic acids chemically change the fxation properties of the soil. They
- neutralize both acid and alkaline soils; regulate the pH-value of soils.
- improve and optimize the uptake of nutrients and water by plants.
- increase buffering properties of soil.
- act as natural chelator for metal ions under alkaline conditions and promote their uptake by the roots.
- rich in both organic and mineral substances essential to plant growth.
- retain water soluble inorganic fertilizers in the root zones and reduce their leaching.
- possess extremely high cation-exchange capacities.
- promote the conversion of nutrient elements (N, P, K + Fe, Zn and other trace elements)
into forms available to plants. - enhance the uptake of nitrogen by plants.
- reduce the reaction of phosphorus with (Ca, Fe, Mg and Al) and liberate it into a form that is available and beneficial to plants. The productivity of particularly mineral fertilizers is increased considerably.
- liberate carbon dioxide from soil calcium carbonate and enable its use in photosynthesis.
- help to eliminate chlorosis caused by iron deficiency in plants.
- reduce the availability of toxic substances in soils.
2.3. Biological Benefits:
Humic acids biologically stimulate the plant and the activities of microorganisms. They
- stimulate plant enzymes and increase their production.
- act as an organic catalyst in many biological processes.
- stimulate growth and proliferation of desirable micro-organisms in soil.
- enhance plants natural resistance against disease and pest.
- stimulate root growth, especially vertically and enable better uptake of nutrients. Increased root respiration and root formation.
- promote the development of chlorophyll, sugars and amino acids in plants and support photosynthesis. Raise in vitamin and mineral content of plants.
- thicken the cell walls in fruits and prolong the storing and shelf time.
- increase in germination and viability of seeds.
- stimulate plant growth (higher biomass production) by accelerating cell division, enhancing the rate of formation in root systems, which results in higher yield of dry matter.
- improve the quality of yields, their physical appearance and nutritional value.
Optimal Utilization of Nutrients
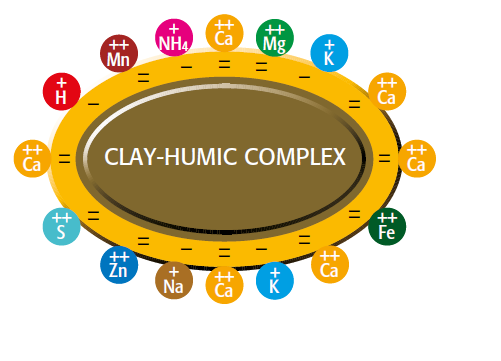 clay-humic complex Increased nutrient deposit by humic acid
clay-humic complex Increased nutrient deposit by humic acid
Figure 2.1 — Increased nutrient deposit
by humic acid.
(Copyright © Humintech 2018)
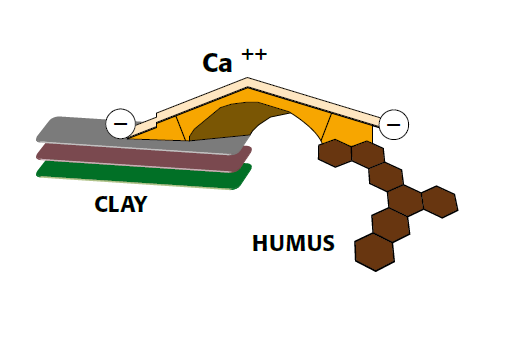 Ca-Bridge between clay and humus
Ca-Bridge between clay and humus
Figure 2.2 — Ca-Bridge between clay and humus.
(Copyright © Humintech 2018)
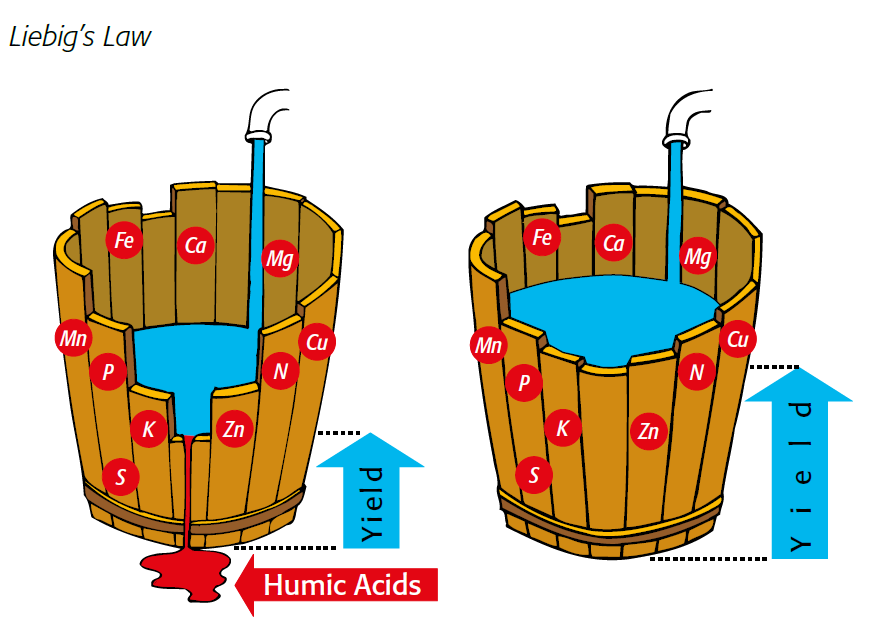 illustration of liebig's law Growth is controlled by the scarcest resource
illustration of liebig's law Growth is controlled by the scarcest resource
Figure 2.3 — Growth is controlled by the scarcest resource.
(Copyright © Humintech 2018)
Effect in soils
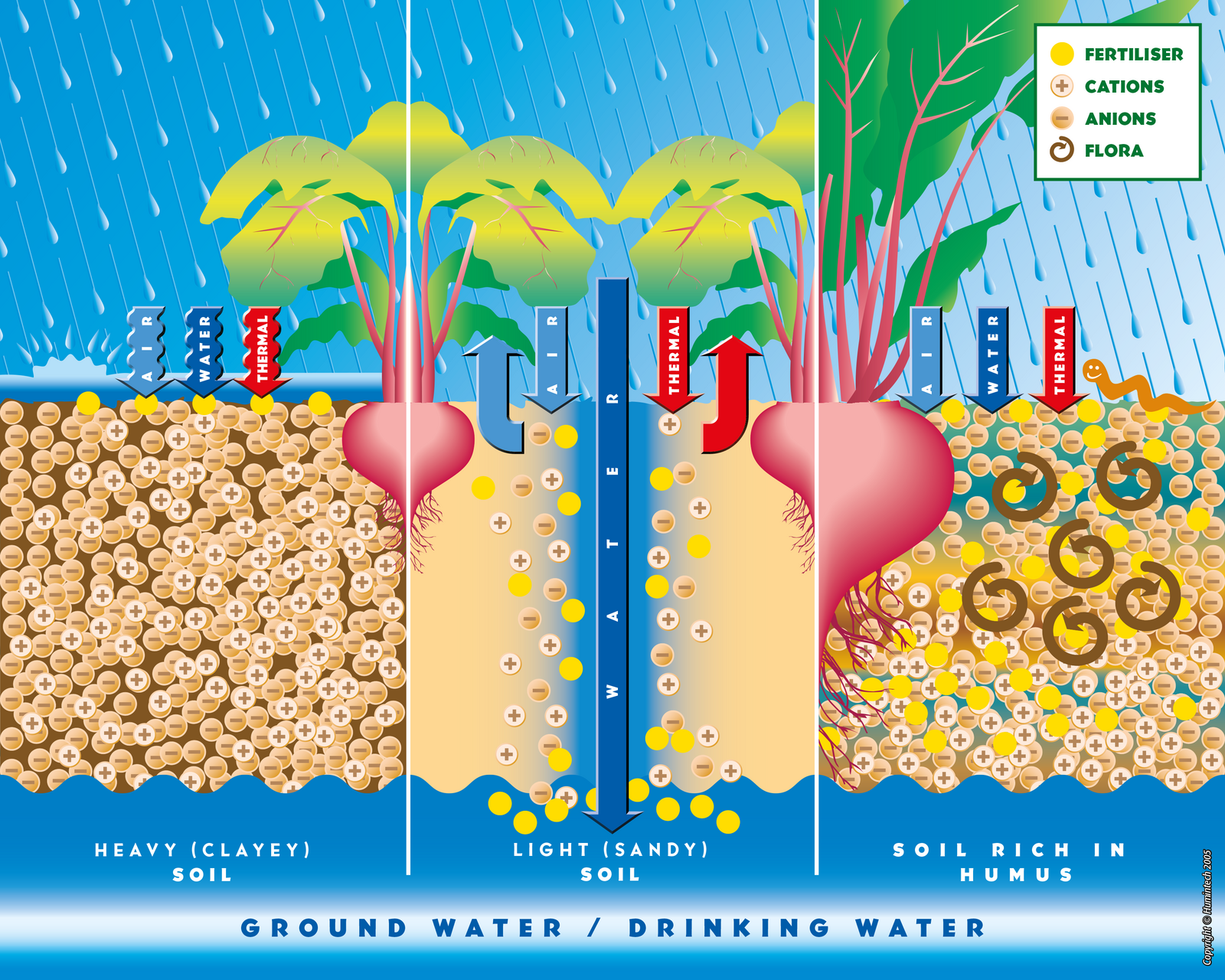
Comparative scheme of heavy clayey, light sandy and rich in humus soils.
(Copyright © Humintech 2005)
Compacted Clay Soils
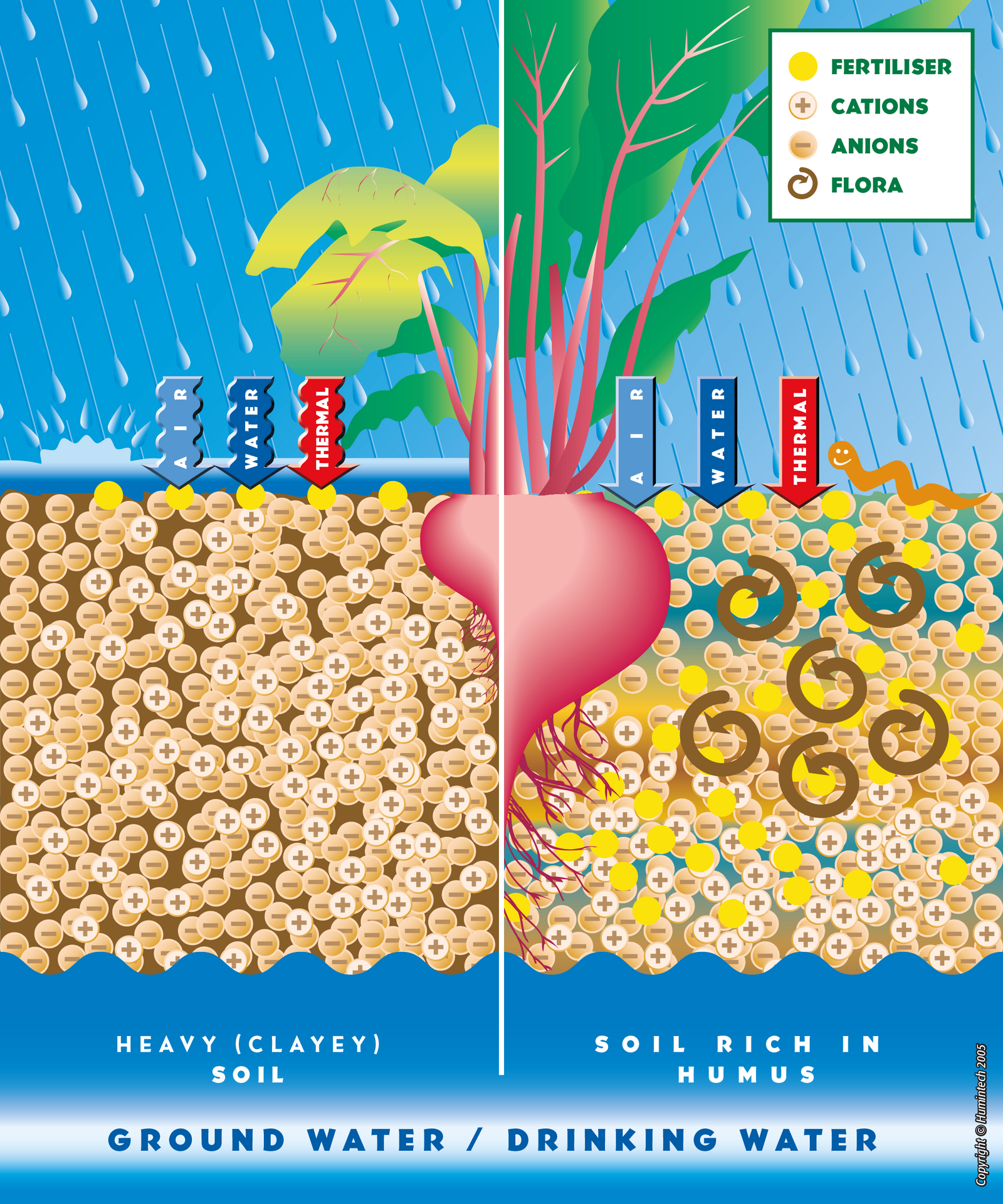
Humic acids aerate compacted soils and improve their structure. Thus water, nutrients and roots can penetrate the soil more easily (s. pic. 2.3, fig. 2.4, 2.5).
Comparative scheme of heavy clayey soils and rich in humus soils.
(Copyright © Humintech 2005)
 Compacted clay soil without humic substance
Compacted clay soil without humic substance
Picture 2.3 - Compacted day soil without humic substance. (Copyright © Humintech 2018)
 Compact, hardly penetrable soil structure
Compact, hardly penetrable soil structure
Figure 2.4 — Compact, hardly penetrable soil structure.
(Copyright © Humintech 2018)
 Humic acids aerate compact soils
Humic acids aerate compact soils
Figure 2.5 — Humic acids aerate compact soils.
(Copyright © Humintech 2018)
Light Sandy Soils
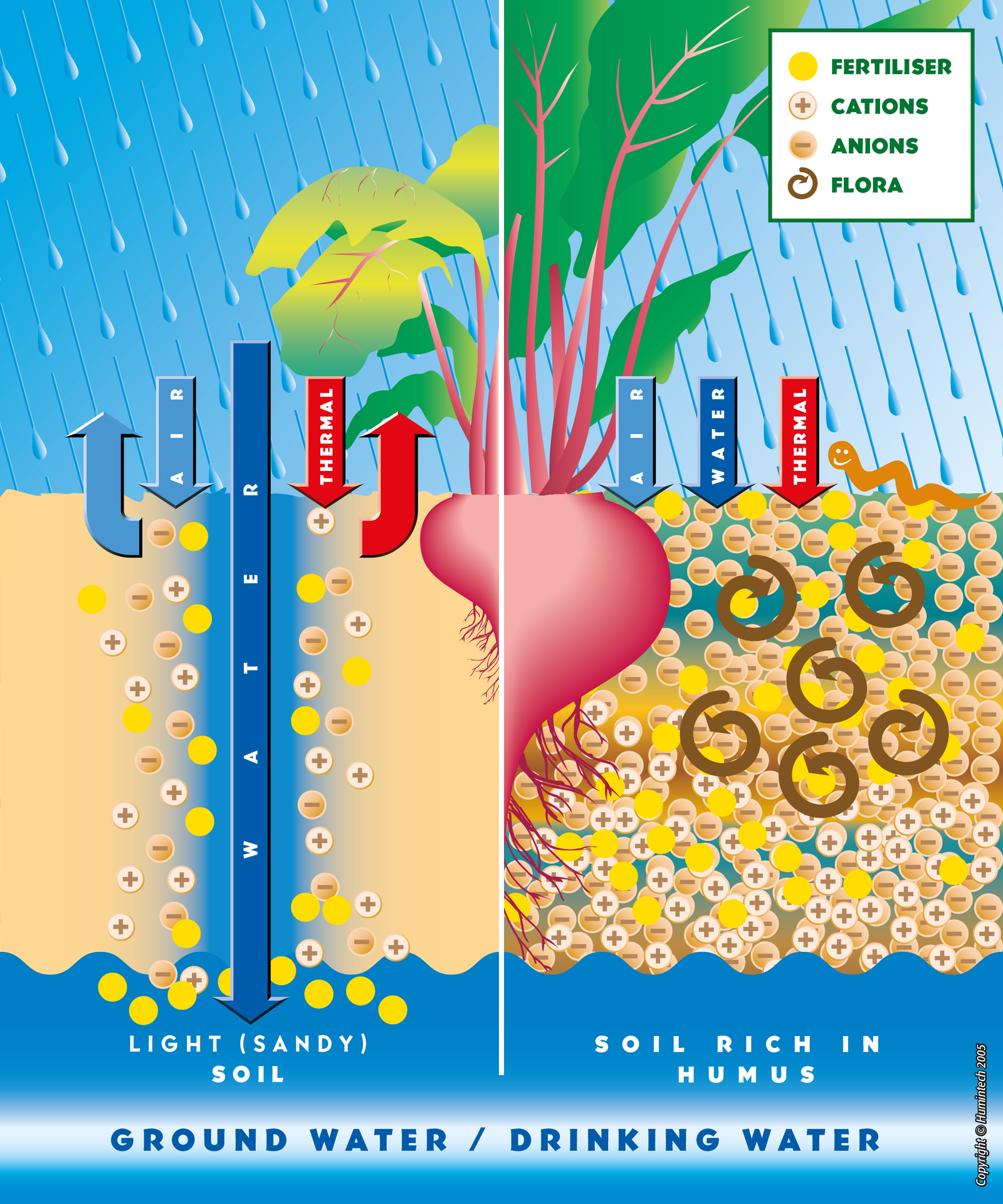
In sandy soils, poor in humus, humic acid coats the sand particles, improves the cation exchange capacity (CEC) and increases the ability of the soil to retain nutrients and water. Therefore, nutrients (particulary nitrate) remain available for the plants (s. pic. 2.1, 2.2, fig. 2.6, 2.7).
Comparative scheme of light sandy soils and rich in humus soils.
(Copyright © Humintech 2005)
 Sandy soils without humic substance
Sandy soils without humic substance
Picture 2.1 - Sandy soils without humic substance
 Greening with PERLHUMUS
Greening with PERLHUMUS
Picture 2.2 - Greening with PERLHUMUS®.
 Sandy soils poor in humus can’t retain nutrients
Sandy soils poor in humus can’t retain nutrients
Figure 2.6 — Sandy soils poor in humus can’t retain nutrients.
(Copyright © Humintech 2018)
 Effect of the cation exchange capacity to sandy soils
Effect of the cation exchange capacity to sandy soils
Figure 2.7 — Effect of the cation exchange
capacity to sandy soils.
(Copyright © Humintech 2018)
Salinized Soils
Salts are split up by the high cation exchange capacity (CEC) of Humic acids. Cations (e.g. Ca and Mg) are bound and chelated. The high osmotic pressure within the root area is reduced.
(s. fig. 2.8, 2.9, 2.10, pic. 2.4).
Humic acid can help improve salinity tolerance in turfgrass by mitigating the negative effects of salt stress on plant growth and development.
It does this by reducing sodium toxicity, increasing potassium uptake, regulating osmotic balance, and enhancing antioxidant enzyme activity. Humic acid can also improve soil structure and water retention, further contributing to salinity tolerance.
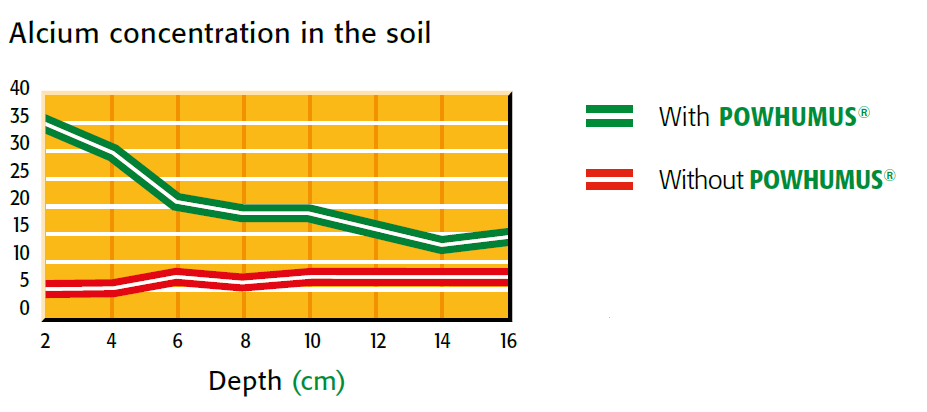 calcium concentration in the soil with and without powhumus
calcium concentration in the soil with and without powhumus
Figure 2.8 — Splitting of salt. (Copyright © Humintech 2018)
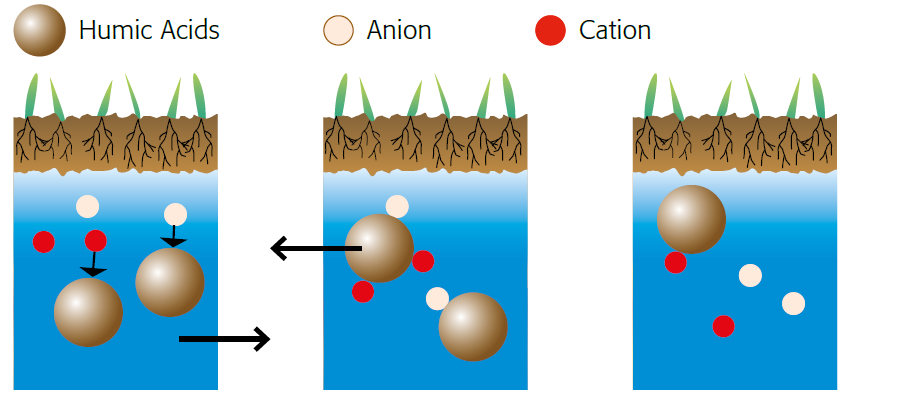 Splitting of salt
Splitting of salt
Figure 2.9 — Humic acids reduce the effects of salinity. (Copyright © Humintech 2018)
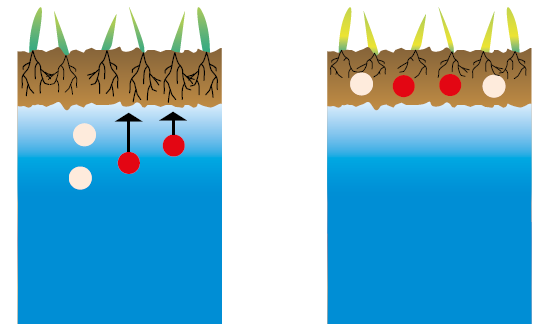 Humic acids reduce the effects of salinity
Humic acids reduce the effects of salinity
Figure 2.10 — Salinized groundwater in a soil. (Copyright © Humintech 2018)
 Highly Salinized soil
Highly Salinized soil
Picture 2.4 — Highly Salinized soil.
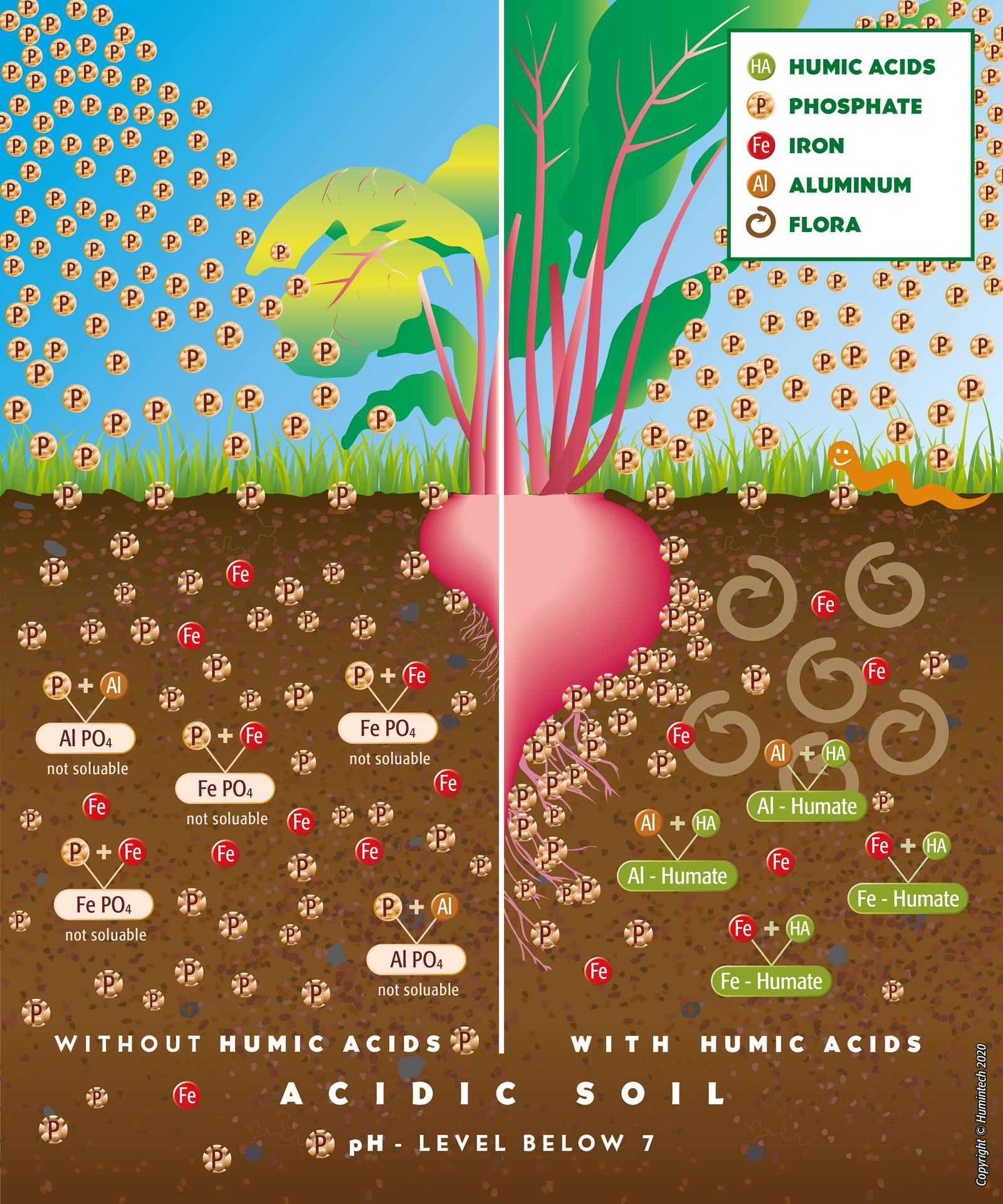
Acidic Soils
Due to their high buffer capacity humic acids neutralize acidic soils, which decimate acid caused stress in plant roots. Elements harmful for plants, especially aluminium and heavy metals, are bound firmly and immobilized by humic acids. Hence, their toxicity is reduced and phosphate bound by aluminium is released (s. fig. 2.11, 2.12).
Figure 2.11 — Comp. scheme of acidic soils without and with humus acids.
(Copyright © Humintech 2020)
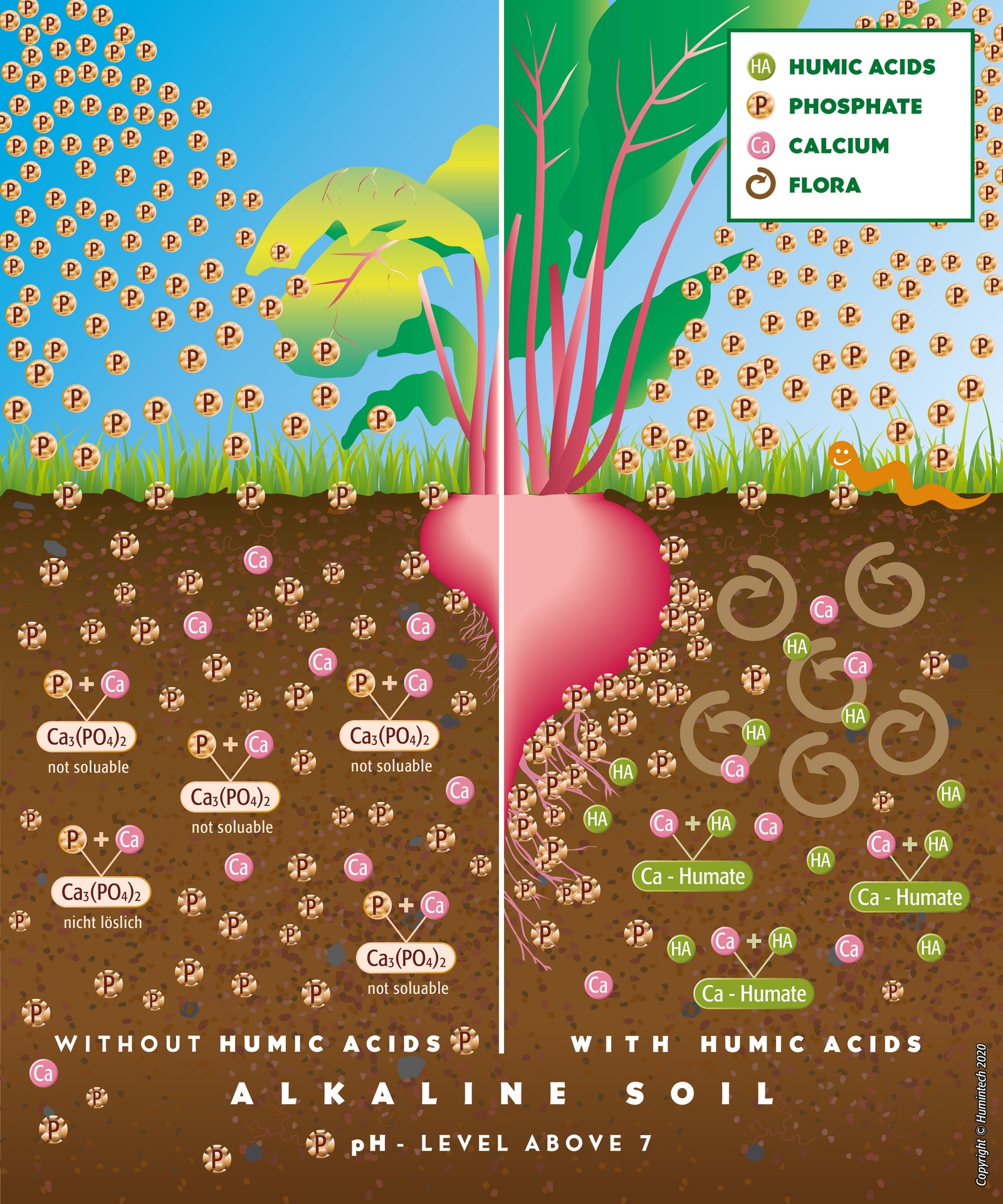
Alkaline Soils
Alkaline Soil As a result of the high pH value, many essential nutrients and trace elements are not in plant available form. Humic acids buffer the high pH and convert nutrients and trace elements into
plant receptible form by complexation. Phosphate bound by calcium is resolubilized and made available (s. fig. 2.13, 2.12).
Figure 2.13 — Comp. scheme of alkaline solis without and with humus acids.
(Copyright © Humintech 2020)
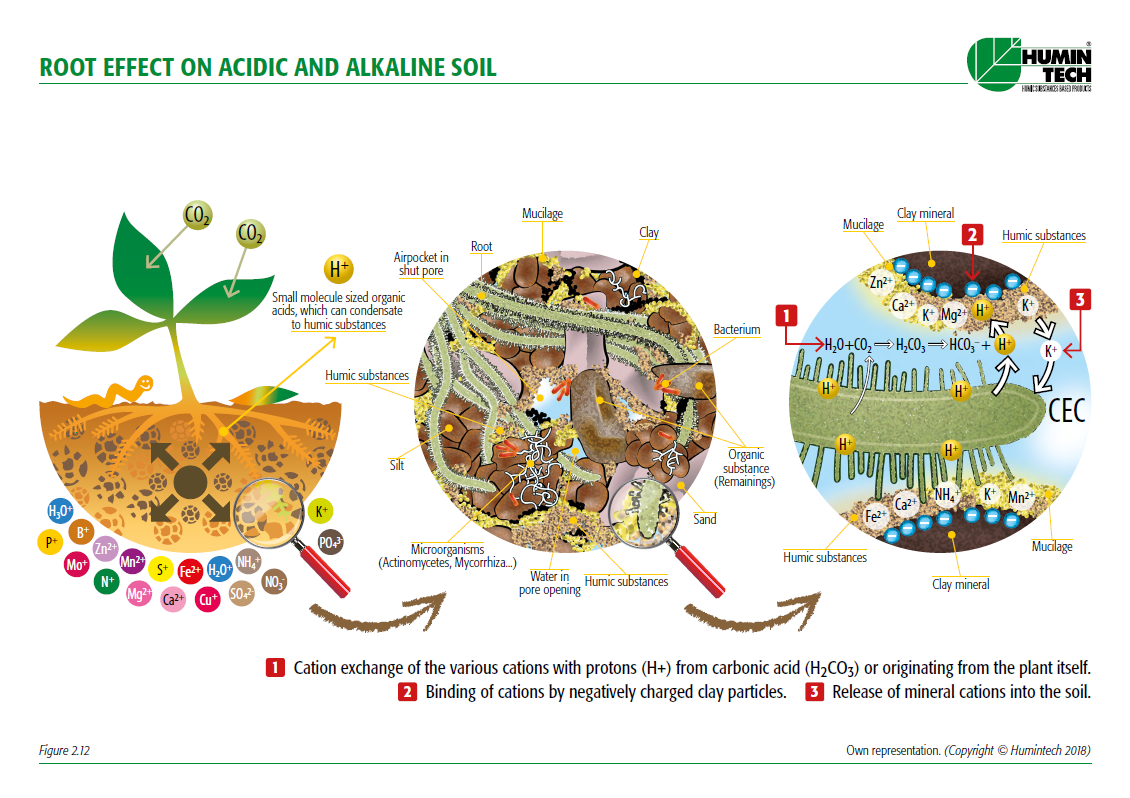
Root efect on acid and alkaline soil
Eroded Soils
The addition of humic acids accumulates the organic matter in the topsoil. The erosion is effectively reduced by intensified root formation and stabilizing clay-humus complexes.
Dry Soils
Humic acids increase the ability of the soil to retain water. Thus, water is available for the plants in dry periods too. That way drought caused stress situations to the plants are avoided and the wastage of precious water is reduced.
Pesticide, Herbicide, and Fungicide Charged Soils
Humic acids raise the efficiency of pesticides, fungicides as well as herbicides and immobilize their harmful residues.
3. ECOLOGICAL BENEFITS OF HUMIC ACIDS
The ecological benefits of humic acids are diverse, present profitable and effective solutions for environmental problems along with the preservation of the environment.
First of all, soils with a high content of humic acids are a guarantee for low nitrate leaching and for optimum nutrient efficiency. A well developed root system, which is achieved by a high content of humic acids, prevents that nitrate and pesticides mix in with ground water (s.fig 3.1). Futhermore, a low content of nitrate is an indicator and a prerequisite for appropriate organic agriculture.
It happens very often that growers use fertilizers more than plants can take up. This leads to nitrate concentration in soil, which is later to be found in ground water. As a result, this contaminated water can only be purified by a complex and expensive wastewater treatment process.
Important: Instead of curing the symptoms (water contamination) only, the basic causes (nitrate leaching) need to be tackled.
Secondly, humic acids reduce the over salination problem in the application of watersoluble mineral fertilizers. Humic acids are able to decrease high salt con-tents in soils and thus the resulting toxicities. Especially the NH4-toxicity of fertilizers containing ammonia is reduced, which is of great importance for young plants particularly.
Generally, humic acids reduce root burning which comes about through excessive salt concentrations in soils after fertilization; in case of permanent high levels of salt in soils, these are reduced. Furthermore, when humic acids are mixed with liquid fertilizers, the undesirable smell is diminished.
Thirdly, humic acids are an effective means to fight against soil erosion. This is achieved by increasing the ability of soil colloids to combine and by enhancing root system and plant development.
Leonardite and humate -based products are certificated for organic agriculture by renown organizations and institutions of agriculture worldwide (s. fig 3.1).
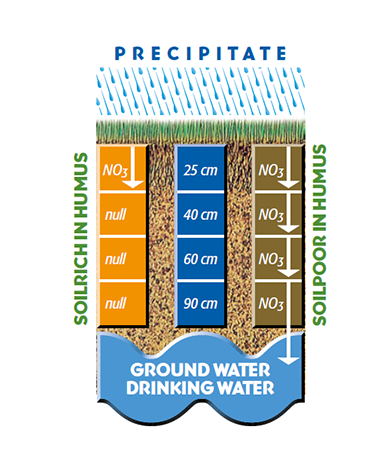
Figure 3.1 — Reduction of nitrate leaching. (Copyright © Humintech 2018)
4. ECONOMIC BENEFITS OF HUMIC ACIDS
Humic acids chelate nutrient compounds, especially iron, to obtain a suitable formfor plant utilization in soli, so the nutrient supply of plants is optimized. High increases up to 70% in yield, accompanied by a reduction up to 30% in the use of fertilizers and pesticides, as well as better and healthier growth of green grass, ornamentals, agricultural crops and woods can be attained with the regular application of first-quality humic acids. Furthermore, water holding capacity of soils is increased considerably, which means that the use of water can be reduced substantially. Best economic results can be obtained in light and sandy soils poor in humus as well as on recultivation fields.
The diverse positive impacts of humic acids are to be observed particularly in such soils. This is true for almost all soils in dry and warm regions. As a result of the high mineralization rate of organic substances, providing these soils with stable humic acids is indispensable for the maintenance and improvement of soil fertility.
5. HUMINTECH
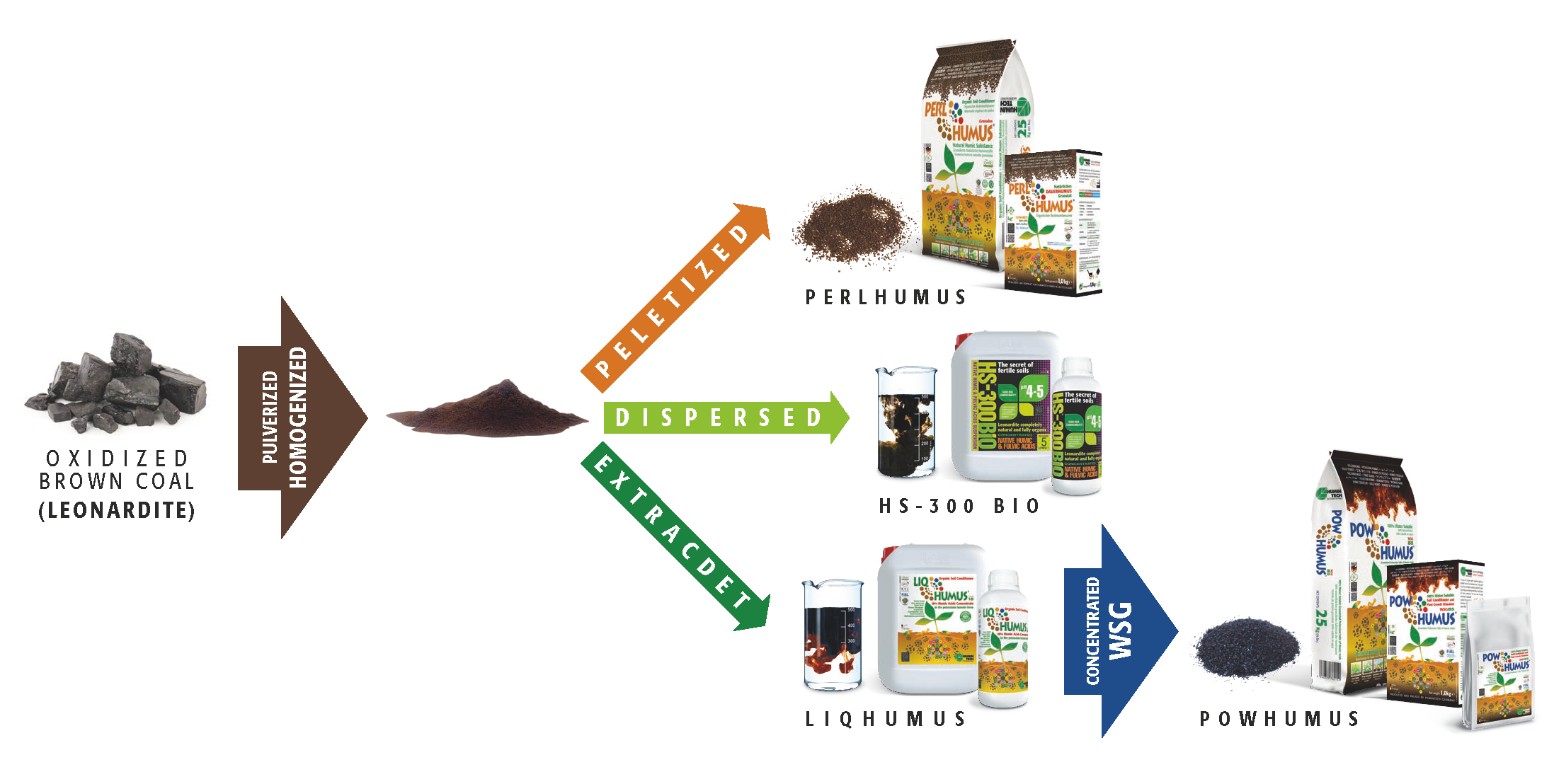
HUMINTECH® offers a variety of products which successfully fulfil the needs of different soil conditions and plants (s. pic.5.1). As a result, soils treated with HUMINTECH® products secure qualitative and quantitative increases in yield and reduce material and labor costs. Made of humic substances of the highest quality, HUMINTECH® extensive product line has been designed to suit the demands of a healthy community.
Picture 5.1 — Some products of the HUMINTECH® product line